Required practical 8
Investigation into the effect of a named factor on the rate of dehydrogenase activity in extracts of chloroplasts.
Overview
- Dehydrogenase is an enzyme in the Light Dependent Reaction
- It catalyses the reaction involved in NADP accepting electrons
- Redox indicators such as DCPIP or methylene blue can be used to investigate the activity of dehydrogenase.
- When DCPIP is oxidised it is blue, when it has accepted electrons it is colourless (reduced)
- We can measure the rate of dehydrogenase activity, by measuring the rate at which the indicator changes from oxidised (blue) to colourless
High Level Method
-
Prepare a chloroplast extract by breaking down the leaf tissue in a suitable buffer and using centrifugation to separate chloroplasts from other cell components.
-
Add a fixed volume of chloroplast extract to a fixed volume of DCPIP in test tubes and mix gently.
-
Expose each test tube to a different light intensity, for example by placing them at different distances from a lamp (e.g. 30 cm, 50 cm, 100 cm)
-
Measure the absorbance of each sample using a colorimeter at regular intervals (e.g. every 2 minutes for 10 minutes).
-
Determine the rate of dehydrogenase activity from the decrease in absorbance of DCPIP over time, comparing rates between light intensities.
Interpreting Results
-
DCPIP is blue when oxidised and has a high absorbance
-
When DCPIP is reduced it becomes colourless, so absorbance decreases
-
The faster DCPIP becomes colourless, the higher the rate of dehydrogenase activity
-
As the reaction occurs in the light-dependent stage of photosynthesis, increasing light intensity increases the rate of electron transfer to DCPIP, so DCPIP decolourises faster
-
Example graph with conditions below
-
A = steepest decrease → fastest rate
- Highest light intensity (e.g nearest to the lamp)
-
B = moderate decrease → medium rate
- Moderate light intensity (e.g not the nearest to the lamp but still receives enough light)
-
C = shallow decrease → slowest rate
- Shade/low light intensity
Exam Question Practice
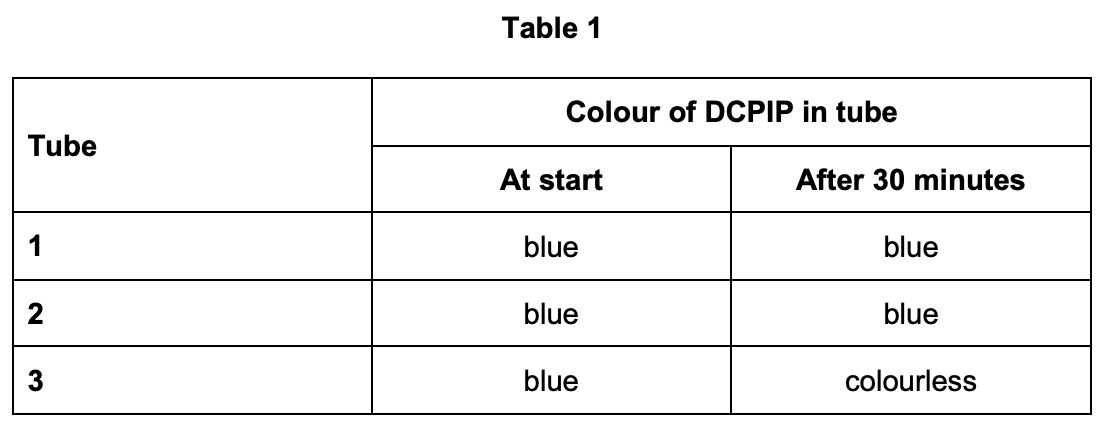
A student isolated chloroplasts from spinach leaves into a solution to form a chloroplast suspension. He used the chloroplast suspension and DCPIP solution to investigate the light-dependent reaction of photosynthesis. DCPIP solution is blue when oxidised and colourless when reduced.
The student set up three test tubes as follows:
- Tube 1 – 1 cm³ of solution without chloroplasts and 9 cm³ of DCPIP solution in light.
- Tube 2 – 1 cm³ of chloroplast suspension and 9 cm³ of DCPIP solution in darkness.
- Tube 3 – 1 cm³ of chloroplast suspension and 9 cm³ of DCPIP solution in light.
The student recorded the colour of the DCPIP in each of the tubes at the start and after the tubes had been left at 20 °C for 30 minutes. His results are shown in Table 1.
The solution that the student used to produce the chloroplast suspension had the same water potential as the chloroplasts.
Explain why it was important that these water potentials were the same.
(2 marks)Hint
What would happen to the chloroplasts if water potentials weren’t kept the same?
Answer
Mark Scheme
- Osmosis does not occur (1 mark)
- Chloroplast/organelle does not burst/lyse/shrivel/shrink (1 mark)
Tips from examiner reports
- 80% students got 1 mark.
- Common errors included discussing the effect of osmosis on ‘the cell’ rather than on chloroplasts and the use of terms such as ‘plasmolysed’ and ‘turgid’.
Explain why the student set up Tube 1.
(2 marks)Hint
What was removed from this tube? Would light alone affect DCPIP?
Answer
Mark Scheme
- To show light does not affect DCPIP (1 mark)
- To show chloroplasts are required (1 mark)
Tips from examiner reports
- <10% of students obtained both marks.
- Common errors were saying that tube 1 was a control.
- Students needed to mention that Tube 1 showed that chloroplasts were needed to cause the colour change, and that light (without chloroplasts) won’t affect DCPIP.
The student evaluated the effectiveness of different chemicals as weed-killers by assessing their ability to prevent the decolourisation of DCPIP in chloroplast suspensions.
Explain what causes the decolourisation of DCPIP.
(2 marks)Hint
Explain what is happening with DCPIP to cause a change in colour, and where does this occur?
Answer
Mark Scheme
- Reduction of DCPIP by electrons (1 mark)
- (From) chlorophyll/light dependent reaction (1 mark)
Tips from examiner reports
- Over 80% of students obtained at least one mark for this question.
- Common misconceptions suggested that DCPIP was reduced by protons or by reduced NADP (or reduced NAD). Or referring to the oxidation of DCPIP by oxygen or the release of ions from chlorophyll.
He added different concentrations of each chemical to illuminated chloroplast suspensions containing DCPIP. He then determined the IC50 for each chemical. The IC50 is the concentration of chemical which inhibits the decolourisation of DCPIP by 50%.
Explain the advantage of the student using the IC50 in this investigation.
(1 marks)Answer
Mark Scheme
- Provides a standard/reference point OR Can compare different chemicals/weedkillers (1 mark)
Explain how chemicals which inhibit the decolourisation of DCPIP could slow the growth of weeds.
(3 marks)Hint
Think about how the light dependent reaction links to the light independent reaction. What are the products of the light dependent reaction, and what would subsequently happen if they weren’t produced?
Answer
Mark Scheme
- Less/no ATP produced (1 mark)
- Less/no reduced NADP produced (1 mark)
- Less/no GP reduced/converted to TP (1 mark)
Tips from examiner reports
- 50% students got this question incorrect.
- Many showed understanding that the light-dependent reaction would not take place, and hence neither would the Calvin cycle. This was then often linked to less glucose being produced for growth.
- However, many of these answers failed to mention the products of the light-dependent reaction.